Due to anthropogenic activities the world’s oceans are changing dramatically. Increasing stratification triggered by a rising sea surface temperature (SST) changes nutrient availability particularly influencing access to the macronutrients phosphorus (P) and nitrogen (N) (Gerace et al., 2025). Additionally, fossil fuel burning releases carbon dioxide (CO2), which partly gets absorbed in the oceans but affects several biogeochemical processes and contributes to ocean acidification (Caldeira and Wicket, 2003; Heinze et al., 2015). These environmental changes are most expressed in open ocean systems (Polovina et al., 2008), home to globally important cyanobacteria like Synechococcus and Prochlorococcus (Visintini et al., 2021), which might be less competitive in a warmer future ocean than previously thought (Ribalet et al. 2025). This indicates the importance to resolve how future ocean condictiones affect the physiology and capability of cyanobacteria to fix carbon dioxide.
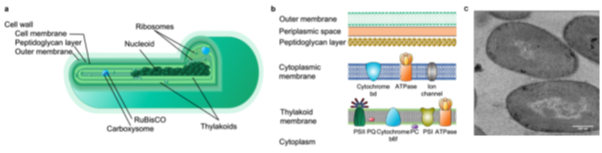
Cells possess lipid membranes as a barrier towards ever changing environments. In phototrophs, lipids further act as a key component of the thylakoid membrane embedding the photosynthetic machinery (Figure 1) and hence are critical for photosynthesis. However, lipid membranes undergo changes due to prevailing environmental conditions. For example, under limiting P concentrations, cells replace phospholipids with P-free lipids to provide P for other cellular functions (Van Mooy et al., 2009). Previous work in our group showed that lipid remodelling induced by P limitation affects carbon fixation in several Synechococcus spp. (Mausz et al. in prep.). Yet the mechanisms behind this process remain unclear as well as what other physiological consequences these remodelled cyanobacteria will face under future ocean conditions and what this means for global carbon fixation.
This project has two major aims:
First it wants to determine eco- and photophysiological consequences of P-limitation induced lipid remodelling in cyanobacteria and study the responsible mechanisms based on transcriptomics data.
Second, it wants to explore how the combination of lipid remodelling and future ocean conditions such as ocean acidification or increasing SST affect cell physiology and carbon fixation rates.
Figure 1: Cyanobacterial cell and its lipid membranes. (a) Scheme of a cyanobacterial cell and (b) its lipid membranes with the different embedded protein complexes in the cytoplasmic and thylakoid membrane. (c) Transmission electron microscopy image of Synechococcus sp. PCC 7002 showing membranes of thylakoid stacks. Scale bar equals 500 nm. Abbreviations: RubisCO, ribulose-1,5-bisphosphate carboxylase/oxygenase; PC, plastocyanin; PQ, plastoquinone; PSI and PSII, photosystem I and II.
This project is not suitable for CASE funding
Each host has a slightly different application process.
Find out how to apply for this studentship.
All applications must include the CENTA application form.
Choose your application route
This project will cultivate the model cyanobacterium Synechococcus sp. PCC 7002 under varying future ocean conditions (i.e., P limitation, high CO2 concentration, increased temperature). To investigate mechanistic changes based on transcriptomic data, a wild type and lipid synthesis knockout mutant pair is available, other mutants will be designed as required and several marine cyanobacteria are available in our laboratory.
Membrane lipid analysis uses in-house liquid chromatography-mass spectrometry (LC-MS) instrumentation. Photophysiology will be studied using a Phyto-PAM Phytoplankton Analyzer (e.g. rapid light curves), radioisotope assays to access CO2 fixation, and a unique Joliot type spectrophotometer (JTS-150) to directly target the performance of photosystem I and II. Nutrient uptake kinetics (methylammonium, phosphate, sulphate) will be analysed by radioisotope assays. An in-house proteomics mass spectrometry facility allows the investigation of protein expression. Morphological changes will be assessed by imaging flow cytometry and various microscopy techniques accessible from Warwick Research Technology Platforms (RTP).
DRs will be awarded CENTA Training Credits (CTCs) for participation in CENTA-provided and ‘free choice’ external training. One CTC can be earned per 3 hours training, and DRs must accrue 100 CTCs across the three and a half years of their PhD.
Training is available in the supervisor’s and co-supervisors’ labs with a special focus on different cultivation approaches for cyanobacteria including the usage of a Qubit Gas Control System for cultivation under high CO2. Training in other microbiological techniques (e.g. cloning, flow cytometry), lipid and protein extraction, lipidomics, proteomics, and transcriptomics analyses, bioinformatics tools, and radioisotope assays are also available. Special importance further has training in the conduction of photophysiology measurements using a Phyto-PAM and JTS-150 with interpretation and analysis of obtained data. Training in imaging techniques and imaging flow cytometry is available via staff at the Warwick RTPs.
This project does not have any external partners, but students will have the opportunity to collaborate with colleagues from the supervisors’ established networks.
Year 1: Characterise effects (photophysiology, carbon fixation, morphology, proteomics, nutrient uptake) of lipid remodelling on Synechococcus sp. PCC 7002
Year 2: Transcriptomic data analysis and set-up of experiments to study mechanistic insights, mutant creation, physiological measurements of combined stressors (e.g. lipid remodelling and temperature).
Year 3: Synechococcus spp. lipid remodelling in a high CO2 world.
Caldeira K, Wicket ME (2003) Anthropogenic carbon and ocean pH. Nature 425: 365.
Gerace SD, Yu J, Moore K, Martiny AC (2025) Observed declines in upper ocean phosphate-to-nitrate availability. Proc. Natl. Acad. Sci. USA 122: e2411835122, https://doi.org/10.1073/pnas.2411835122.
Heinze C, Meyer S, Goris N, Anderson L, Steinfeldt R, Chang N, Le Quéré C, Bakker DCE (2015) The ocean carbon sink – impacts, vulnerabilities and challenges. Earth Syst. Dynam. 6: 327-358.
Polovina JJ, Howell EA, Abecassis M (2008) Ocean’s least productive waters are expanding. Geophys. Res. Lett. 35: L03618, https://doi.org/10.1029/2007GL031745.
Ribalet F, Dutkiewicz S, Monier E, Armbrust EV (2025) Future ocean warming may cause large reductions in Prochlorococcus biomass and productivity. Nat. Microbiol. https://doi.org/10.1038/s41564-025-02106-4
Van Mooy BAS, Fredricks HF, Pedler BE, Dyhrman ST, Karl DM, Koblížek M, Lomas MW, Mincer TJ, Moore LR, Moutin T, Rappé MS, Webb EA (2009) Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 458, 69-72.
Visintini N, Martiny AC, Flombaum P (2021) Prochlorococcus, Synechococcus, and picoeukaryotic phytoplankton abundances in the global ocean. Limnol. Oceanogr. Lett. 6: 207-215.
Applicants should hold a BSc and/or MSc degree or equivalent in a relevant subject. Informal enquiries can be directed to Dr Michaela Mausz ([email protected]).
To apply to this project:
Applications must be submitted by 23:59 GMT on Wednesday 7th January 2026.