This PhD project aims to develop a human thyroid–liver organ-on-a-chip (OoC) platform to investigate how environmental chemical pollutants disrupt endocrine function, with a focus on thyroid hormone disruption. By integrating omics and systems toxicology approaches, the project will provide a physiologically relevant, mechanistic alternative to animal-based testing, directly addressing the environmental challenge of precisely assessing endocrine-disrupting chemicals (EDCs) released into air, water, soil, and food chains. The key research questions include: 1) How can a microphysiological thyroid–liver chip be optimised to capture hormone production, transport, and metabolism in a human-relevant context that reflects environmentally mediated exposure? 2) Can this platform accurately model disruption of thyroid hormone homeostasis by environmental contaminants, including mixtures, and identify mechanistic biomarkers of endocrine disruption? 3) How can omics and systems toxicology approaches be integrated to predict environmentally relevant adverse outcomes and support next-generation chemical safety assessment?
To achieve these goals, the project will develop thyroid organoids with follicular structure and thyroid-stimulating hormone (TSH)-dependent hormone production, co-cultured with liver organoids capable of phase I/II metabolism and thyroid hormone clearance. These models will be integrated into a microfluidic chip to simulate inter-organ crosstalk and dynamic hormone regulation. A panel of environmentally relevant chemicals and mixtures will be applied to evaluate dose–response effects and mechanisms of thyroid disruption. High-throughput omics technologies (e.g. transcriptomics, metabolomics, proteomics) will characterise molecular responses.
The anticipated outcomes of this research include the development of a reproducible and mechanistically informative thyroid–liver chip that can serve as a viable New Approach Methodology (NAM) for environmental toxicology, advancing the 3Rs (Replacement, Reduction, Refinement) in animal use. Academically, the project will contribute to organ-on-chip innovation, endocrine disruption research, and systems-level toxicology, generating high-impact publications and international conference presentations. Beyond academia, the platform will have significant regulatory and industrial applications, with potential use in environmental toxicology, environmental risk assessment, and policy development for pollution control.
The project will be structured for 3.5 years: the first year will focus on literature review, organoid optimisation, and chip design; the second year will involve establishing co-culture models, functional validation, and chemical testing; and the final year will emphasise omics integration, systems modelling, regulatory discussions, and thesis preparation. By the end of the PhD, this research aims to deliver a validated thyroid–liver chip platform for predictive, mechanistically informed assessment of environmental endocrine disruptors.
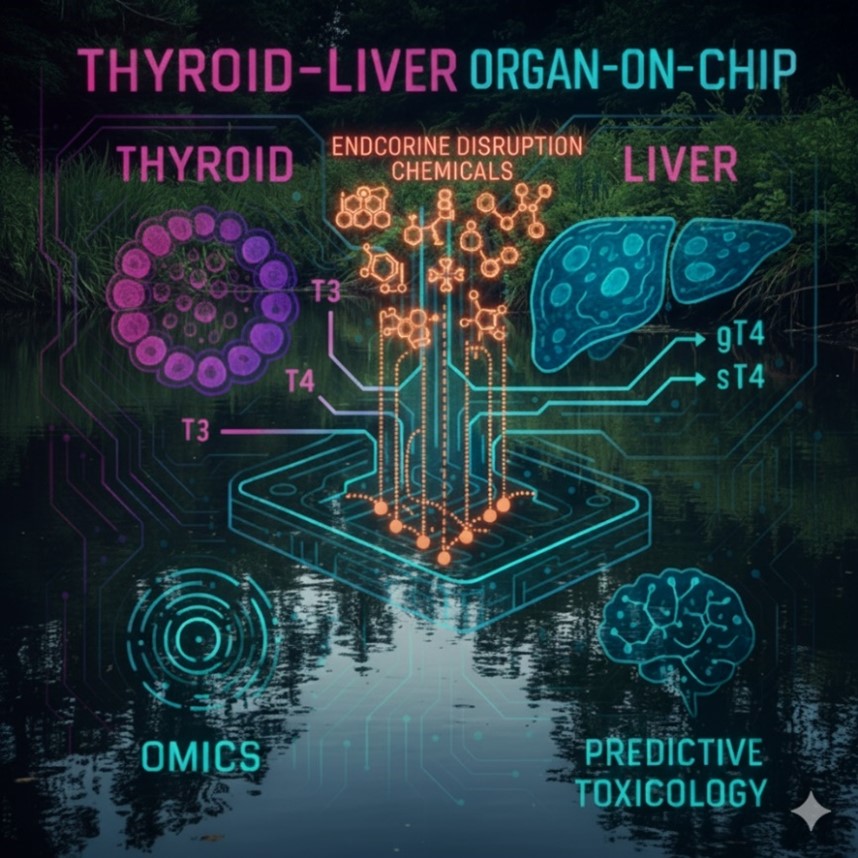
Figure 1: Thyroid-Liver Organ-on-Chip Platform for Assessment of Thyroid Disruption Induced by Environmental Pollutants.
This project is a CENTA Flagship Project.
This project is suitable for CASE funding
Each host has a slightly different application process.
Find out how to apply for this studentship.
All applications must include the CENTA application form.
Choose your application route
Human thyroid organoids will be developed to form follicular structures with TSH-dependent triiodothyronine (T3) and thyroxine (T4) production. In parallel, liver organoids (HepaRG or iPSC-derived hepatocytes) will be optimised to provide thyroid hormone clearance through phase I/II metabolism, generating glucuronidated and sulfated thyroxine. These modules will be integrated into a microfluidic chip under dynamic flow to replicate physiological crosstalk between hormone synthesis, binding, and metabolism. A panel of environmental chemicals and mixtures, will be applied to evaluate dose–response effects on hormone homeostasis. High-throughput omics approaches (e.g. transcriptomics, metabolomics, proteomics) will characterise molecular pathways of disruption. Finally, systems toxicology modelling, such as AOPs, will integrate data into predictive frameworks for environmental risk assessment.
DRs will be awarded CENTA Training Credits (CTCs) for participation in CENTA-provided and ‘free choice’ external training. One CTC can be earned per 3 hours training, and DRs must accrue 100 CTCs across the three and a half years of their PhD.
The student will gain interdisciplinary training in environmental science, toxicology, and data science. Experimental skills will include organoid culture, organ-on-chip engineering, and multi-omics profiling to study how environmental pollutants disrupt thyroid regulation. Computational training will focus on systems toxicology, data integration, and predictive modelling using frameworks such as Adverse Outcome Pathways (AOPs). The student will also learn to translate mechanistic findings into regulatory and environmental risk assessment contexts. Professional development will be supported through collaboration with academic and industrial partners, providing transferable skills for careers in environmental toxicology, science-policy, and sustainable chemical safety.
Dr Helen Tinwell (Bayer Crop Science) will act as industrial Co-Investigator, contributing expertise in endocrine disruption testing and regulatory toxicology. Bayer will advise on chemical selection and provide regulatory insight to align the platform with OECD/EFSA guidance. The Bayer Data Science team will deliver training and guidance in systems toxicology modelling and data integration. Collaboration will involve co-supervision, quarterly project reviews, and a CASE placement (Year 2/3) offering hands-on experience in regulatory toxicology, assay translation, and computational modelling. This partnership ensures project outputs are scientifically rigorous, regulatory relevant, and impactful for both academia and industry.
Kühnlenz J, Karwelat D, Steger-Hartmann T, et al. A microfluidic thyroid-liver platform to assess chemical safety in humans[J]. ALTEX-Alternatives to animal experimentation, 2023, 40(1): 61-82.
Schneider MR, Oelgeschlaeger M, Burgdorf T, van Meer P, Theunissen P, Kienhuis AS, Piersma AH, Vandebriel RJ. Applicability of organ-on-chip systems in toxicology and pharmacology. Crit Rev Toxicol. 2021 Jul;51(6):540-554.
Jia X, Wang T, Zhu H. Advancing Computational Toxicology by Interpretable Machine Learning. Environ Sci Technol. 2023 Nov 21;57(46):17690-17706.
For any enquiries related to this project please contact Pu Xia, [email protected]
To apply to this project:
Applications must be submitted by 23:59 GMT on Wednesday 7th January 2026.