Sudden environmental changes are challenging for the survival of many organisms. Some organisms evolved mechanisms to cope with uncertainty, by sensing the environment and transmitting selected adaptive traits to the next generation.
We use the nematode Auanema freiburgense as model to study the mechanisms by which environmental signals sensed by the mother results in the modification of the germline to produce stress-resistant progeny. In this nematode, chemicals produced by nematodes of the same species are used as signals for overcrowding. Thus, by sensing these chemicals, the mother ‘prepares’ the progeny to withstand the lack of food that occurs in overcrowded conditions. The progeny arrests development in the form of larvae, and can survive in the absence of food for several months. Once in a benign environment, the larvae resume development to become self-fertilizing adults. The main objectives of the project are to identify the chemical nature sensed by the mothers, how the sensory neurons convey the information to the gonad, and how the germline changes result in different kinds of progeny.
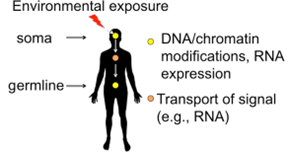
Figure 1: Conceptual framework. An environmental trigger changes the state of the soma, which sends a signal to modify the germline.
This project is not suitable for CASE funding
Each host has a slightly different application process.
Find out how to apply for this studentship.
All applications must include the CENTA application form.
Choose your application route
Chemicals will be isolated from nematode cultures and tested for their influence on the sex determination and stress-resistance in the F1 generation. To identify the neuron sensing the chemicals, single cells will be tested by killing them with the use of a laser microbeam. The nature of the communication signal between the neuron and the germline will be tested by performing gene knockouts using the genome editing technology. Changes in the germline upon neuronal signal will be tested using immunoprecipitation with antibodies recognizing histone modification markers.
Research involving nematodes is generally considered to fall outside the scope of traditional vertebrate animal ethics regulations (e.g., APHA/Home Office in the UK for vertebrates). This is because nematodes are invertebrates lacking a central nervous system and nociceptors (pain receptors) comparable to those of vertebrates, meaning they are not considered sentient and do not experience pain or distress in the same way.
DRs will be awarded CENTA Training Credits (CTCs) for participation in CENTA-provided and ‘free choice’ external training. One CTC can be earned per 3 hours training, and DRs must accrue 100 CTCs across the three and a half years of their PhD.
Students will learn to use the latest genome editing technologies (CRISPR-Cas9) to inactivate gene function and to tag genes to visualize their time and site of expression. Furthermore, students will acquire skills in bioinformatics (learn how to code in Unix and R), how to ablate single cells and immunocytochemistry. In addition, students will learn how to organize and execute their experiments in a timely fashion, how to document experiments, prepare presentations, write professional articles and work in a team. Many of those skills are transferable to other disciplines and professions.
Not applicable.
Year 1: Fraction and test chemicals produced by the nematode. Make laser ablations of single neurons.
Year 2: Generate mutants for neuroamines and neuropeptides using CRISPR/Cas9. Characterize mutants. Start immunocoprecipitation experiments.
Year 3: Transcriptome analysis of animals exposed to defined chemicals. Characterise candidate genes involved in cross-generational inheritance using genome editing technologies
For any enquiries related to this project please contact Andre Pires da Silva, University of Warwick, [email protected], https://olive-finch.lnx.warwick.ac.uk/Pires/
To apply to this project:
Applications must be submitted by 23:59 GMT on Wednesday 7th January 2026.