Project highlights
- In collaboration with BIFoR this project will test the effect of future atmospheric CO2 on oak reproduction and to what extent oak will become better or less-well adapted to that environment.
- This project will identify genes that might control oak puberty and reproduction as a route to speed-breeding, and whether the lifecycle of oak can be accelerated by manipulating environmental conditions
- In collaboration with RGB Kew this project will map the natural sequence diversity of these genes within oaks and assess the future importance of these variants for oak breeding programs.
Overview
Oak (Quercus robur) is an ecological keystone tree species within the UK, with each tree a habitat for up to 200 other species (Mitchell et al., 2019). However, plant reproduction is highly environmentally-sensitive, with some tree species already responding to climate change by altering the time at which they flower (Büntgen et al., 2022), risking the potential loss of entire species if plant-pollinator interactions are disrupted or trees cannot otherwise successfully reproduce. Oak is remarkably long-lived and slow-growing compared to its close relatives, taking 25-40 years to begin flowering. Today’s generation of oaks will thus bear the brunt of environmental changes without time to naturally adapt to the challenges this will present. The potential risk of climate change to oak must therefore be taken seriously, but large-scale experiments such as the Free Air Carbon Enrichment (FACE) study being conducted by the Birmingham Institute for Forest Research (BIFoR) allow us to simulate future atmospheric conditions and predict the challenges to oak reproduction in the future.
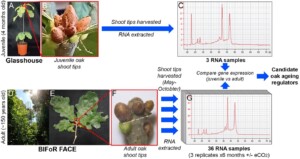
We do not understand why oak reproduces so slowly. Plant ageing has been studied only in fast-growing annual plants such as the laboratory model Arabidopsis thaliana (Wu et al., 2009). Genes with the potential to control ageing and flowering in oaks have been identified from preliminary gene expression data (Figure 1), but their functions remain untested. This project will improve our understanding of oak ageing and reproduction in the following ways:
- Determine how future atmospheric CO2 concentrations will affect ageing mechanisms in oak saplings and reproductive success in mature oaks, utilizing BIFoR FACE.
- Test if oak ageing and flowering mechanisms are similar to those from Arabidopsis using wet-lab experiments, and whether ‘speed-breeding’ by altering growth conditions (Watson et al., 2018) can potentially be implemented in oak.
- 3. Utilising the Oak 2000 genomes dataset of RGB Kew, map the natural variation of candidate ageing genes within the oak population to quantify oak’s capacity for adaptation through breeding programmes.
Figure 1: Oak candidate ageing regulators have been identified by quantifying and comparing gene expression between shoot tips of juvenile saplings (A-C) and adult trees (D-G).
Host
University of BirminghamTheme
- Organisms and Ecosystems
Supervisors
Project investigator
- Andrew Plackett, University of Birmingham, [email protected]
Co-investigators
- Graeme Kettles, University of Birmingham, [email protected]
- Richard Buggs, Royal Botanic Gardens, Kew, [email protected]
How to apply
- Each host has a slightly different application process.
Find out how to apply for this studentship. - All applications must include the CENTA application form. Choose your application route
Methodology
Previously-identified candidate oak ageing and flowering genes will be validated by real-time PCR (qPCR) analysis of expression using a 3-year library of samples already available in the lab.
To test if the same mechanisms exist between oak and the model plant Arabidopsis thaliana, the student will genetically engineer Arabidopsis to insert oak versions of known ageing genes and study their function.
The student will help to set up an oak transformation system based on previously-published tissue culture techniques and somatic embryogenesis (Álvarez & Ordás 2007) to create genetically engineered oak.
The student will conduct glasshouse experiments to test the effect of environmental conditions on oak ageing.
The student will use bioinformatics to analyse the natural variation in gene sequences between the genomes of individual oak trees, in collaboration with Prof Richard Buggs. The student will also conduct bioinformatic analysis of RNA-seq data generated from their glasshouse experiments.
Training and skills
DRs will be awarded CENTA Training Credits (CTCs) for participation in CENTA-provided and ‘free choice’ external training. One CTC can be earned per 3 hours training, and DRs must accrue 100 CTCs across the three and a half years of their PhD.
Training in all project-specific techniques can be provided by the project supervisors. Prof Richard Buggs can provide support with bioinformatic analyses. Dr Graeme Kettles can provide training and support and with qPCR. Dr Andrew Plackett can provide training and support in tissue culture, the genetic engineering of Arabidopsis and field-sampling at the FACE experiment. Other transferrable-skills training is available via the University of Birmingham.
It would be advantageous for the student to have some prior experience with bioinformatic analysis and general wet-lab experience with molecular biology techniques. A valid driver’s licence accepted within the UK would also be an advantage.
Partners and collaboration
Richard Buggs is a Professor of Evolutionary Genomics at the Queen Mary University London, and leads the Plant Health and Adaptation research team at Royal Botanic Gardens Kew. His research group analyses genome sequences to understand how plants, especially trees, adapt in response to climate change and new pests and pathogens. This project will access the 2000 oak genomes datasets belonging to the Buggs Group to asses the natural variation in specific candidate genes. It is envisaged that this project will be based primarily at the University of Birmingham Edgbaston campus with Prof Buggs providing advice and support remotely.
Further details
For further details and discussion about this project, please contact Dr Andrew Plackett at the University of Birmingham School of Biosciences, email address [email protected].
To apply to this project:
- You must include a CENTA studentship application form, downloadable from: CENTA Studentship Application Form 2025.
- You must include a CV with the names of at least two referees (preferably three) who can comment on your academic abilities.
- Please submit your application and complete the host institution application process via: https://sits.bham.ac.uk/lpages/LES068.htm. Please select the PhD Bioscience (CENTA) 2025/26 Apply Now button. The CENTA Studentship Application Form 2025 and CV can be uploaded to the Application Information section of the online form. Please quote CENTA 2025-B25 when completing the application form.
Applications must be submitted by 23:59 GMT on Wednesday 8th January 2025.
Possible timeline
Year 1
Screen candidate oak ageing genes by qPCR using existing tissue samples. Identify and clone oak versions of Arabidopsis genes for use in genetic engineering. Perform bioinformatic analysis to assess the natural variation of oak candidate genes in the 2000 oak genomes dataset. Set up and test oak tissue-culture protocol.
Year 2
Create genetically-engineered Arabidopsis plants carrying oak versions of ageing genes and establish pure-breeding lines for analysis. Attempt genetic engineering of oak. Begin glasshouse experiments testing CO2 enrichment and/or speed-breeding conditions on oak saplings.
Year 3
Test genetically-engineered Arabidopsis lines for oak gene functions in controlling ageing. Test genetically-engineered oak saplings for accelerated ageing by qPCR or RNA-seq. Complete analysis of glasshouse environmental manipulation.
Further reading
Journals:
Álvarez, R. and Ordás, J. J. (2007) ‘Improved genetic transformation protocol for cork oak (Quercus suber L.)’, Plant Cell, Tissue and Organ Culture, 91, pp. 45-52. doi: 10.1007/s11240-007-9276-6.
Büntgen, U., Piermattei, A., Krusic, P. J., Esper, J., Sparks, T., and Crivellaro, A. (2022) ‘Plants in the UK flower a month earlier under recent warming’, Proceedings of the Royal Society B., 289, article 20212456. doi: 10.1098/rspb.2021.24.
Mitchell, R. J., Bellamy, P. E., Ellis, C. J., Hewison, R. L., Hodgetts, N. G., Iason, G. R., et al. (2019) ‘Collapsing foundations: The ecology of the British oak, implications of its decline and mitigation options’, Biological conservation, 233, pp. 316-327. doi: 10.1016/j.biocon.2019.03.040
Watson, A., Ghosh, S., Williams, M.J., Cuddy, W.S., Simmonds, J., Ray, M.D., et al. (2018). ‘Speed breeding is a powerful tool to accelerate crop research and breeding’, Nature Plants, 4, pp. 23-29. DOI: 10.1038/s41477-017-0083-8.
Wu, G., Park, M. W., Conway, S. R., Wang, J. W., Weigel, D., and Poethig, R. S. (2009) ‘The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis’, Cell, 138, pp. 750-759. DOI: 10.1016/j.cell.2009.06.031.
Web-page:
BIFoR FACE experiment. Available at:
https://www.birmingham.ac.uk/research/bifor/face/index.aspx (Accessed: 17 September 2024)