Project highlights
- Utilisation of compounds derived from metal waste recycling directly in catalysis, to progress towards a circular economy.
- Development of greener electrolyte systems using Deep Eutectic Solvents (DES), to reduce waste and pollution.
- Testing catalyst systems for CO2 capture and conversion to high-value products, powered by renewable electrical energy.
Overview
Carbon dioxide (CO2) levels on Earth have reached an all-time high, and mitigating anthropogenic (man-made) climate change is a defining challenge of our era. However, the global economy and our society are critically dependent on fossil fuels, which meet 80% of the world’s energy demands and feed the production of 95% of all chemical commodities, we rely on in our everyday lives. Nature uses CO2 as its primary one-carbon building-block for biomass and now the chemical industry is beginning to realise its potential as a cheap, renewable feedstock for the production of vital chemicals.
Conversion of CO2 is challenging as the molecule is chemically very stable, and a vast energy input is required to make it react. Catalysts are needed to lower this energy barrier and transition metals (such as copper and nickel) present the best sites for CO2 activation. However, mining of these metals has significant environmental impacts which need to be addressed for a truly sustainable process to be developed at scale. Furthermore, although an overwhelming number of catalysts have been developed to generate one-carbon products from CO2, there are very few examples in which multi-carbon (C2+) products are formed. This presents an opportunity with massive potential impact for the sustainable chemicals industry, since 2-carbon compounds like ethylene present the best trade-off between high economic value and a reduction in global warming potential, if they could be produced from CO2 using renewable electrical energy.
Altering the composition of the solvent-electrolyte media is one of the main approaches for improving overall catalyst efficiency in the electrochemical CO2 conversion. Ionic liquids (ILs) are promising in this regard, but there are issues with their ecotoxicity and non-biodegradability. Natural deep eutectic solvents (DES) present a more sustainable alternative to ILs in that they are non-toxic, and highly bio-compatible. DES have been successfully applied in metal-catalysed and biocatalysed reactions, however, their potential in the catalytic conversion of CO2 to high-value chemicals has yet to be realised.
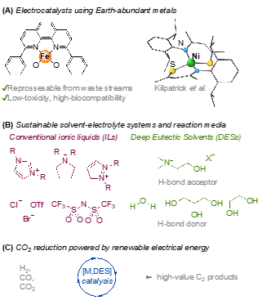
Figure 1. Harnessing renewable energy with captured CO2 for the chemical value chain.
Case funding
This project is suitable for CASE funding
Host
University of LeicesterTheme
- Climate and Environmental Sustainability
Supervisors
Project investigator
Dr Sandy Kilpatrick, School of Chemistry, University of Leicester, [email protected]
Co-investigators
Dr Phil Bird, Argo Natural Resources (trading as Descycle)
Prof. Karl Ryder, Centre for Sustainable Materials Processing, University of Leicester, [email protected]
How to apply
- Each host has a slightly different application process.
Find out how to apply for this studentship. - All applications must include the CENTA application form. Choose your application route
Methodology
The student will carry out synthesis and characterisation of catalysts based on Earth-abundant metals in the Kilpatrick group laboratories (Figure 1A). The potential for recovering the active metals (e.g. nickel, iron) from primary mining waste steams will be investigated.
Deep Eutectic Solvents (DES) will be investigated as a more “green” solvent-electrolyte media, while maintaining high catalyst efficiency and stability (Figure 1B). The performance of DES in catalytic CO2 conversion will be probed via analytical and bulk electrochemical experiments. Initial studies will employ known molecular catalysts, in combination with DES, both neat and as an additive in various solvents (water, acetonitrile, propylene carbonate, 2-(2-ethoxyethoxy)ethanol).
This CENTA project aims to validate a hypothesis that by creating an increased local CO2 concentration and stabilising key intermediates, carbon-carbon bond forming reactions will be promoted. Accordingly, a new class of sustainable catalysts for the conversion of CO2 into economically valuable C2+ products will be developed (Figure 1C).
Training and skills
Training and skills:
Students will be awarded CENTA2 Training Credits (CTCs) for participation in CENTA2-provided and ‘free choice’ external training. One CTC equates to 1⁄2 day session and students must accrue 100 CTCs across the three years of their PhD.
The successful candidate will gain extensive experience of anaerobic protocols used in catalyst synthesis (Schlenk and glovebox techniques), electrochemical CO2 reduction (cyclic voltammetry, chronoamperometry), as well as product detection and quantification (spectroscopic and analytical techniques).
The candidate will also gain experience in innovative metal recovery and recycling methods using eco-friendly DES, skills for evaluating the environmental impact of the catalyst, and knowledge of the commercial landscape through collaboration with the company Argo Natural Resources (trading as Descycle).
The supervisory team will establish CENTA-specific training sessions to facilitate knowledge of the current carbon capture and utilisation state-of-the-art, and potentially disruptive CO2 conversion technologies for Net Zero chemicals.
Partners and collaboration
Dr Sandy Kilpatrick is a Lecturer and early career researcher with interests in Sustainable Synthesis and Catalysis, in particular applying novel electrochemical techniques to CO2 reduction.
Dr Phil Bird is a Scientific Officer and Mineral Processing Specialist at Argo Natural Resources (TA Descycle).
Prof. Karl Ryder is a leading researcher in novel electrolyte media.
This is a new interdisciplinary collaboration, combining distinct and complementary expertise and skillsets towards a common goal of disruptive sustainable chemical technologies. By these means, we can realise the vision of a net zero future, producing platform chemicals from CO2 for the coming decades, and beyond. Potential future commercialisation of the technology/methods will be developed by the CENTA student.
Further details
Further details on how to contact the supervisor for this project and how to apply for this project can be found here:
Potential applicants are welcome to discuss the project informally with the project supervisors:
Dr Sandy Kilpatrick https://www.kilpatrickgroup.uk/ (research group website) https://le.ac.uk/people/alexander-kilpatrick (UoL staff pages)
Dr Phil Bird https://descycle.com (company website)
Prof. Karl Ryder https://le.ac.uk/people/karl-ryder (UoL staff pages)
To apply to this project:
- You must include a CENTA studentship application form, downloadable from: CENTA Studentship Application Form 2024.
- You must include a CV with the names of at least two referees (preferably three) who can comment on your academic abilities.
- Please submit your application and complete the host institution application process via: https://le.ac.uk/study/research-degrees/funded-opportunities/centa-phd-studentships. Please scroll to the bottom of the page and click on the “Apply for NERC CENTA Studentship” button. Your CV can uploaded to the Experience section of the online form, the CENTA application form 2024 can be uploaded to the Personal Statement section of the online form. Please quote CENTA 2024-L2-CENTA2-CHEM2-KILP when completing the application form.
Applications must be submitted by 23:59 GMT on Wednesday 10th January 2024.
Possible timeline
Year 1
Synthesis and characterisation of catalysts for CO2 conversion featuring Earth-abundant metals. Investigating extraction of catalytic metals (e.g. iron, nickel) from primary mining waste streams. Gas reactions and in situ spectroscopy giving detailed understanding of CO2 activation mechanisms.
Year 2
Engineering of a solvent-electrolyte system: development of biocompatible ionic liquids and natural deep eutectic solvents, and testing their suitability for in CO2 electrocatalysis.
Year 3
Combining catalyst and DES solvent-electrolyte to deliver a scalable electrocatalyst system for CO2 conversion to value-added products, with low overpotential and high selectivity identified.
Further reading
Chen, Y. and Mu, T. (2019) ‘Conversion of CO2 to value-added products mediated by ionic liquids’, Green Chemistry, 21(10), pp. 2544–2574. doi: 10.1039/c9gc00827f.
Grim, R. G. et al. (2020) ‘Transforming the carbon economy: Challenges and opportunities in the convergence of low-cost electricity and reductive CO2 utilization’, Energy and Environmental Science, 13(2), pp. 472–494. doi: 10.1039/c9ee02410g.
Smith, E. L., Abbott, A. P. and Ryder, K. S. (2014) ‘Deep Eutectic Solvents (DESs) and Their Applications’, Chemical Reviews, 114(21), pp. 11060–11082. doi: 10.1021/cr300162p.
Vasilyev, D. V et al. (2019) ‘A General and Facile Approach for the Electrochemical Reduction of Carbon Dioxide Inspired by Deep Eutectic Solvents’, ChemSusChem, 12(8), pp. 1635–1639. doi: 10.1002/cssc.201900579.
Zhang, J. et al. (2023) ‘Molecular tuning for electrochemical CO2 reduction’, Joule, 7(8), pp. 1700–1744. doi: 10.1016/j.joule.2023.07.010.