Project highlights
- The project aims to address the remediation of greenhouse gases, specifically focusing on the decomposition of nitrous oxide (N2O).
- The studentship will explore photochemical promoted heterogeneous catalysis, harnessing solar energy to enhance the catalytic activity of metal oxide materials. This approach offers potential for mitigating N2O emissions and reducing its impact as a potent greenhouse gas.
- Working collaboratively with supervisors and project partners, the student will develop various catalyst targets. This will foster innovation and creativity in catalyst design, contributing to the advancement of sustainable solutions for greenhouse gas remediation.
Overview
Nitrous oxide (N2O) is a potent greenhouse gas, with a global warming potential 300 times greater than carbon dioxide, and the dominant ozone depleting substance emitted in the 21st century. Although naturally occurring, increasing anthropogenic N2O emissions from intensive agricultural fertilisation, industrial processes, and combustion of fossil fuels and biomass are a major cause for concern due to the detrimental impact this will have on our environment. The selective decomposition of N2O into environmentally benign N2 and O2 is a compelling prospect to remediate such emissions but, despite being a thermodynamically favourable reaction, activation of this weakly interacting triatomic gas is encumbered by a considerable degree of kinetic inertness. The overarching aim of this project is to harness solar energy to promote this reaction, using thermally and industrially relevant metal oxide materials as catalysts.
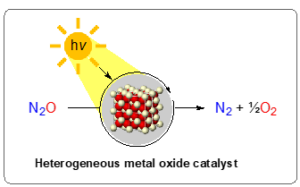
Figure 1: Schematic overview of project aim: the photochemical promoted decomposition of nitrous oxide into dinitrogen and dioxygen using a heterogenous metal oxide catalyst
Host
University of WarwickTheme
- Climate and Environmental Sustainability
Supervisors
Project investigator
Adrian Chaplin; Department of Chemistry, University of Warwick; [email protected]
Co-investigators
Co-I: Richard Walton; Department of Chemistry, University of Warwick;
Co-I: Ryan Mushinski; School of Life Sciences, University of Warwick; [email protected]
How to apply
- Each host has a slightly different application process.
Find out how to apply for this studentship. - All applications must include the CENTA application form. Choose your application route
Methodology
Decomposition of N2O can be promoted thermally using metal oxides but typically requires temperatures >400 °C that are not desirable from a remediation perspective. Photocatalytic alternatives that harness solar energy are attractive and potentially viable alternative but are poorly developed. This project will explore this possibility and involve (a) synthesis of established and novel metal-oxide-based photocatalysts, (b) development of analytical procedures for accurately evaluating N2O decomposition reactions under different regimes (pure N2O, N2O and inert gas mixtures, and dilute N2O in air), and thereafter (c) systematic evaluation of the photocatalysts for N2O decomposition. Precisely monitoring the decomposition of N2O into N2 and O2 is considerable analytical challenge, and a range of techniques will be explored to determine which is best for a given reaction regime. The use of quantitative head space sampling by gas chromatography will be fully explored in combination with FTIR-based methods.
Training and skills
Students will be awarded CENTA2 Training Credits (CTCs) for participation in CENTA2-provided and ‘free choice’ external training. One CTC equates to 1⁄2 day session and students must accrue 100 CTCs across the three years of their PhD.
This PhD project will provide exceptional interdisciplinary training. In addition to the analytical techniques highlighted above, the student working on this project will develop methods for the synthesis and characterisation of technologically relevant solid-state materials; the latter benefiting from the extensive range of analytical techniques available at Warwick such as X-ray diffraction, thermal analysis, and microscopy, with the possibility of developing operando versions of these to understand the mechanism of catalysis and stability of catalysts.
Partners and collaboration
Results from the project will be used to engage with relevant industries, working with Warwick Innovations to protect any emerging Intellectual Property.
Further details
Further details on how to contact the supervisor for this project and how to apply for this project can be found here:
For any enquiries related to this project please contact Adrian Chaplin, [email protected].
To apply to this project:
- You must include a CENTA studentship application form, downloadable from: CENTA Studentship Application Form 2024.
- You must include a CV with the names of at least two referees (preferably three) who can comment on your academic abilities.
- Please submit your application and complete the host institution application process via: https://warwick.ac.uk/fac/sci/lifesci/study/pgr/studentships/nerccenta/ Complete the online application form – selecting course code P-C1PB (Life Sciences PhD); from here you will be taken through to another screen where you can select your desired project. Please enter “NERC studentship” in the Finance section and add Nikki Glover, [email protected] as the scholarship contact. Please also complete the CENTA application form 2024 and submit via email to [email protected]. Please quote CENTA 2024-W1 here when completing the application form.
Applications must be submitted by 23:59 GMT on Wednesday 10th January 2024.
Possible timeline
Year 1
Synthesis of established metal-oxide-based photocatalysts; optimisation of analytical procedures for analysing gaseous mixture of N2O, N2, and O2; analysis of established metal-oxide-based photocatalysts for the decomposition of pure N2O.
Year 2
Iterative preparation, catalytic evaluation, and mechanistic investigation of novel metal-oxide-based photocatalysts for the decomposition of pure N2O and inert gas mixtures of N2O.
Year 3
Continued iterative preparation, catalytic evaluation, and mechanistic investigation of novel metal-oxide-based photocatalysts for the decomposition of inert gas mixtures of N2O, and increasingly dilute mixture of N2O in air.
Further reading
Ravishankara, A.R., Daniel, J.S. & Portmann, R.W., 2009. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science, 326(5949), pp.123–125.
Hansen, J. & Sato, M., 2004. Greenhouse gas growth rates. Proceedings of the National Academy of Sciences of the United States of America, 101(46), pp.16109–16114.
Severin, K., 2015. Synthetic chemistry with nitrous oxide. Chemical Society Reviews, 44(17), pp.6375–6386.
Gyton, M.R., Leforestier, B. & Chaplin, A.B., 2019. Rhodium(I) Pincer Complexes of Nitrous Oxide. Angew. Chem. Int. Ed, 58(43), pp.15295–15298.
Konsolakis, M., 2015. Recent Advances on Nitrous Oxide (N2O) Decomposition over Non-Noble-Metal Oxide Catalysts: Catalytic Performance, Mechanistic Considerations, and Surface Chemistry Aspects. ACS Catalysis, 5(11), pp. 6397–6421.
Ming, T. et al., 2016. Fighting global warming by greenhouse gas removal: destroying atmospheric nitrous oxide thanks to synergies between two breakthrough technologies. Environ. Sci. Pollut. Res., 23(7), pp. 6119–6138.