Project highlights
- The project aims to address the remediation of greenhouse gases, specifically focusing on the hydrogenation of nitrous oxide which yields dinitrogen and water.
- Working collaboratively with the supervisors, you will design, synthesis, characterise, and evaluate new catalysts for the hydrogenation of nitrous oxide.
- This project provides exceptional interdisciplinary training; incorporating the synthesis, characterisation, and catalytic evolution of molecular organometallic complexes and extended polycrystalline materials.
Overview
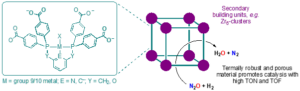
Nitrous oxide (N2O, laughing gas) is an abundant gas that accumulates in the Earth’s atmosphere, leading to depletion of ozone in the stratosphere and contributing to global warming as a potent greenhouse gas. Although naturally occurring, anthropogenic N2O emissions from intensive agricultural fertilisation, industrial processes, and combustion of fossil fuels and biomass are a major cause for concern due to the detrimental impact this will have on our environment. The degradation, reduction, and/or application of nitrous oxide in synthesis have consequently drawn attention. Hydrogenation of nitrous oxide using dihydrogen, which liberates dinitrogen and water, is considered an attractive reductive process but is poorly developed and not currently technologically viable. Known heterogenous processes are characterised by undesirably forcing reaction conditions, whilst the current state-of-the art homogenous catalysts do not have practically useful productivity (ca. limited to <1000 catalytic turnovers). Building upon unpublished breakthrough findings in the Chaplin group, using late transition metal pincer complexes as hydrogenation catalysts, and exploiting the expertise of the Walton group for working with metal organic frameworks (MOFs), we seek to develop highly porous, group 9/10 metal-based MOFs as catalysts for the hydrogenation of nitrous oxide using dihydrogen (Figure 1). These targets are novel 3D functional materials, which marry the advantages of homogenous and heterogenous catalysts for the purpose of reducing nitrous oxide under mild conditions with high fidelity.
Figure 1: Proposed MOF catalysts for the catalytic hydrogenation of nitrous oxide.
Host
University of WarwickTheme
- Climate and Environmental Sustainability
Supervisors
Project investigator
- Prof. Adrian Chaplin, University of Warwick, [email protected]
Co-investigators
- Prof. Richard Walton, University of Warwick, [email protected]
How to apply
- Each host has a slightly different application process.
Find out how to apply for this studentship. - All applications must include the CENTA application form. Choose your application route
Methodology
Benefiting from literature precedent for the incorporation of pincer complexes into metal-organic frameworks, this proposal will focus on optimisation of the synthetic procedures for incorporating suitably reactive precious metal derivatives (e.g. reactive X ligand in Figure 1) and thereafter tuning the associated organometallic chemistry through subtle variations of the supporting pincer ligand (e.g. E and Y donors in Figure 1). Based on preliminary work carried out using non-immobilised, molecular pincer complexes, initial efforts will focus on M = Rh examples. All new MOF will be extensively characterised using the suite of advanced analytical facilities available onsite at Warwick. Catalytic activity will by systematically evaluated, aided by bespoke headspace gas-phase analysis facilities available within the Chaplin group, to correlate structure with activity. These measurements will be contextualised by comparison to molecular analogues and the combined findings will be harnessed to propel subsequent catalyst design and optimisation.
Training and skills
DRs will be awarded CENTA Training Credits (CTCs) for participation in CENTA-provided and ‘free choice’ external training. One CTC can be earned per 3 hours training, and DRs must accrue 100 CTCs across the three and a half years of their PhD.
This project will provide exceptional interdisciplinary training, comprising advanced methods for the synthesis of organometallic compounds and extended polycrystalline materials, along with use of wide range of modern analytical techniques. The latter include diffraction methods (powder/single crystal X-ray diffraction, three-dimensional electron diffraction) in combination with solid-state MAS NMR spectroscopy to establish structure and bulk purity, which would be complemented by small angle X-ray scattering and electron microscopy studies to understand the bulk material properties and homogeneity (dispersity of particle size and shape). All the necessary specialist training will be provided throughout the supervisors and senior members of their research teams.
Partners and collaboration
Results from the project will be used to engage with relevant industries, working with the University of Warwick’s technology transfer office (Warwick Innovations) to review and protect any emerging Intellectual Property.
Further details
Enquires are welcomed and applications should be directed to Dr Adrian Chaplin ([email protected]); please include a current CV that details any past research work.
To apply to this project:
- You must include a CENTA studentship application form, downloadable from: CENTA Studentship Application Form 2025.
- You must include a CV with the names of at least two referees (preferably three) who can comment on your academic abilities.
- Please submit your application and complete the host institution application process via: https://warwick.ac.uk/fac/sci/lifesci/study/pgr/studentships/nerccenta/ University of Warwick projects will be added here: https://warwick.ac.uk/fac/sci/lifesci/study/pgr/studentships/nerccenta/studentships/ and application guidance is at the bottom of this page. Complete the online application form – selecting course code P-C1PB (Life Sciences PhD); from here you will be taken through to another screen where you can select your desired project. Please enter “NERC studentship” in the Finance section and add Nikki Glover, [email protected] as the scholarship contact. Please also complete the CENTA Studentship Application Form 2025 and submit via email to [email protected]. Please quote CENTA 2025-W7 when completing the application form.
Applications must be submitted by 23:59 GMT on Wednesday 8th January 2025.
Possible timeline
Year 1
Synthesis and characterisation of known late transition metal pincer complexes and functionalised MOF and thereafter evaluation in hydrogenation catalysis. Training.
Year 2
Synthesis of new, bespoke variations and evaluation of their catalytic activity. Optimisation of reaction conditions and detailed mechanistic analysis.
Year 3
Year 3 and 4: Continued mechanism lead catalyst development, with a focus on application in flow.
Further reading
Burgess, S.A., Kassie, A., Baranowski, S.A., Fritzsching, K.J., Schmidt-Rohr, K., Brown, C.M., and Wade, C.R. (2016) “Improved Catalytic Activity and Stability of a Palladium Pincer Complex by Incorporation into a Metal–Organic Framework”. Journal of the American Chemical Society 138 (6), 1780–1783
Severin, K., 2015. Synthetic chemistry with nitrous oxide. Chemical Society Reviews, 44(17), pp.6375–6386.
Wu, X., Du, J., Gao, Y., Wang, H., Zhang, C., Zhang, R., He, H., Lu, G. (Max), and Wu, Z. (2024) “Progress and Challenges in Nitrous Oxide Decomposition and Valorization”. Chemical Society Reviews 53 (16), 8379–8423.
Zeng, R., Feller, M., Ben-David, Y., and Milstein, D. (2017) “Hydrogenation and Hydrosilylation of Nitrous Oxide Homogeneously Catalyzed by a Metal Complex”. Journal of the American Chemical Society 139 (16), 5720–5723